Seguridad e inmunogenicidad en lechones con el candidato vacunal E2-CD154, una vacuna de subunidades contra la peste porcina clásica. Resultados del ensayo clínico de fase III
Contenido principal del artículo
Resumen
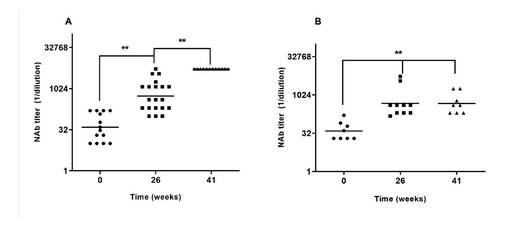
El candidato vacunal E2-CD154 es una vacuna de subunidad cuya seguridad y capacidad protectora en crías frente a la peste porcina clásica ha sido demostrada en estudios previos. Esos hallazgos se confirman en el presente estudio de fase III realizado en dos unidades de producción porcina en la provincia de Pinar del Rio con un número mayor de animales. Todos los animales en ambas granjas recibieron dos dosis de E2-CD154 los días 0 y 21. El estudio se extendió por 60 semanas, la vacuna fue bien tolerada en las crías vacunadas entre los 15 y 28 días de edad, no se documentaron efectos adversos locales o sistémicos. Las cerdas gestantes inmunizadas fueron capaces de trasmitir altos títulos de anticuerpos a sus crías (Unidad A, media geométrica = 1:1295, valor mínimo 1:100 y Unidad B, media geométrica = 1:474, valor mínimo 1:150), muy por encima del umbral de protección (1:50). Estos elevados títulos de anticuerpos neutralizantes derivados de la madre no interfirieron con la inmunogenicidad del candidato vacunal en estudio. Todas las crías vacunadas estudiadas al azar (más del 10% de las 2804) desarrollaron títulos de anticuerpos neutralizantes mayores de 1:400 en los cuatro muestreos realizados (nueve, 21, 41, y 44 semanas) en ambas granjas. Los resultados de este estudio confirman la seguridad, inmunogenicidad y robustez del candidato vacunal en esta sensible categoría en condiciones de campo.
Detalles del artículo

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que prohíbe el uso comercial de sus publicaciones y permite a terceros compartir la obra siempre que se indique su autor y la primera publicación en esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
Citas
Ganges L, Crooke HR, Bohórquez JA, Postel A, Sakoda Y, Becher P, et al. Classical swine fever virus: The past, present and future. Virus research. 2020;289:198151.
Lamothe-Reyes Y, Bohórquez JA, Wang M, Alberch M, Pérez-Simó M, Rosell R, et al. Early and Solid Protection Afforded by the Thiverval Vaccine Provides Novel Vaccination Alternatives Against Classical Swine Fever Virus. Vaccines. 2021;9(5):464.
Li F, Li B, Niu X, Chen W, Li Y, Wu K, et al. The Development of Classical Swine Fever Marker Vaccines in Recent Years. Vaccines. 2022;10(4):603.
Postel A, Austermann-Busch S, Petrov A, Moennig V, Becher P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transboundary and emerging diseases. 2018;65:248-61.
Coronado L, Perera CL, Rios L, Frías MT, Pérez LJ. A Critical Review about Different Vaccines against Classical Swine Fever Virus and Their Repercussions in Endemic Regions. Vaccines. 2021;9(2):154.
Bohórquez JA, Wang M, Díaz I, Alberch M, Pérez-Simó M, Rosell R, et al. The FlagT4G Vaccine Confers a Strong and Regulated Immunity and Early Virological Protection against Classical Swine Fever. Viruses. 2022;14(9):1954.
Choe S, Kim J-H, Kim K-S, Song S, Kang W-C, Kim H-J, et al. Impact of a live attenuated classical swine fever virus introduced to Jeju island, a CSF-free area. Pathogens. 2019;8(4):251.
Coronado L, Rios L, Frías MT, Amarán L, Naranjo P, Percedo MI, et al. Positive selection pressure on E2 protein of classical swine fever virus drives variations in virulence, pathogenesis and antigenicity: Implication for epidemiological surveillance in endemic areas. Transboundary and emerging diseases. 2019;66(6):2362-82.
Jang G, Kim JA, Kang WM, Yang HS, Park C, Jeong K, et al. Endemic outbreaks due to the re-emergence of classical swine fever after accidental introduction of modified live LOM vaccine on Jeju Island, South Korea. Transboundary and emerging diseases. 2019;66(2):634-9.
Sang HJ, Kwon T, Yoo SJ, Lee D-U, Lee S, Richt JA, et al. Classical swine fever outbreak after modified live LOM strain vaccination in naive pigs, South Korea. Emerging infectious diseases. 2018;24(4):798.
Suárez M, Sordo Y, Prieto Y, Rodríguez MP, Méndez L, Rodríguez EM, et al. A single dose of the novel chimeric subunit vaccine E2-CD154 confers early full protection against classical swine fever virus. Vaccine. 2017.
Sordo-Puga Y, Suárez-Pedroso M, Naranjo-Valdéz P, Pérez-Pérez D, Santana-Rodríguez E, Sardinas-Gonzalez T, et al. Porvac(r) Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus. Vaccines. 2021;9(2):167.
Muñoz-González S, Sordo Y, Pérez-Simó M, Suarez M, Canturri A, Rodriguez MP, et al. Efficacy of E2 glycoprotein fused to porcine CD154 as a novel chimeric subunit vaccine to prevent classical swine fever virus vertical transmission in pregnant sows. Veterinary microbiology. 2017;205:110-6.
Sordo-Puga Y, Pérez-Pérez D, Montero-Espinosa C, Oliva-Cárdenas A, Sosa-Teste I, Duarte CA, et al. Immunogenicity of E2CD154 Subunit Vaccine Candidate against Classical Swine Fever in Piglets with Different Levels of Maternally Derived Antibodies. Vaccines. 2021;9(1):7.
Oliva-Cárdenas A, Fernández-Zamora F, Santana-Rodríguez E, Sordo-Puga Y, Vargas-Hernández M, Rodríguez-Moltó M, et al. Safety and immunogenicity in piglets of two immunization schedules initiated at two or three weeks of age with PorvacÒ, a classical swine fever subunit marker vaccine. Bionatura. 2021;6(3).
Pérez-Pérez D, Sordo-Puga Y, Rodríguez-Moltó MP, Sardina T, Santana E, Montero C, et al. E2-CD154 vaccine candidate is safe and immunogenic in pregnant sows, and the maternal derived neutralizing antibodies protect piglets from classical swine fever virus challenge. Veterinary Microbiology. 2021:109153.
VICH. Target Animal Safety for Veterinary live and inactivated Vaccines, GL44. 2010.
Lopez O, editor. Manual De Procedimientos Te´cnicos Para La Crianza Porcina. La Habana, Cuba: Instituto de Investigaciones Porcinas : CIMA.; 2008.
Santana-Rodríguez E, Méndez-Orta M, Sardina-González T, Rodríguez-Moltó M, Castell-Brizuela S, Sordo-Puga Y, et al. Consistency of the Neutralizing Peroxidase Linked Assay for Classical Swine Fever and Homologation with an OIE Reference Laboratory. International Journal of Scientific Research in Biological Sciences. 2022;9(2):30-4.
Biront P, Leunen J, Vandeputte J. Inhibition of virus replication in the tonsils of pigs previously vaccinated with a Chinese strain vaccine and challenged oronasally with a virulent strain of classical swine fever virus. Veterinary microbiology. 1987;14(2):105-13.
Bouma A, de Smit AJ, de Kluijver EP, Terpstra C, Moormann RJ. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet Microbiol. 1999;66(2):101-14.
Terpstra C, Wensvoort G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol. 1988;16(2):123-8.
Parchariyanon S, Tantaswasdi U, Pinyochon W, Methiyapun P. Immunity against swine fever vaccine. II. Immunity against swine fever vaccine in piglets and protection level of maternal immunity in piglets before vaccination J Thai Vet Med Assoc. 1994;45(2):37-45.
Lipowski A, Drexler C, Pejsak Z. Safety and efficacy of a classical swine fever subunit vaccine in pregnant sows and their offspring. Veterinary microbiology. 2000;77(1-2):99-108.
Brun A, Barcena J, Blanco E, Borrego B, Dory D, Escribano JM, et al. Current strategies for subunit and genetic viral veterinary vaccine development. Virus Res. 2011;157(1):1-12.
Lim SI, Song JY, Kim J, Hyun BH, Kim HY, Cho IS, et al. Safety of classical swine fever virus vaccine strain LOM in pregnant sows and their offspring. Vaccine. 2016;34(17):2021-6.
Jackson PG, Cockcroft PD. Handbook of pig medicine: Elsevier Health Sciences; 2007.
Perri AM, O'Sullivan TL, Harding JC, Wood RD, Friendship RM. Hematology and biochemistry reference intervals for Ontario commercial nursing pigs close to the time of weaning. The Canadian Veterinary Journal. 2017;58(4):371.
Muirhead MR, Alexander TJ. Managing pig health and the treatment of disease: A reference for the farm: 5M Enterprises Ltd., PO Box 233.; 1997.
Reinoso Espin LV. Evaluación de la influencia del jugo de caña y un núcleo proteico en el perfil hepático en cerdos en etapa de crecimiento 2014.
Meyer DJ, Harvey JW. Veterinary Laboratory Medicine: Interpretation & Diagnosis: Saunders; 2004.
Chen J-Y, Wu C-M, Chen Z-W, Liao C-M, Deng M-C, Chia M-Y, et al. Evaluation of classical swine fever E2 (CSF-E2) subunit vaccine efficacy in the prevention of virus transmission and impact of maternal derived antibody interference in field farm applications. Porcine health management. 2021;7(1):1-14.