Safety and immunogenicity in piglets of the vaccine candidate E2-CD154, a subunit vaccine against classical swine fever. Results from phase III clinical trial
Main Article Content
Abstract
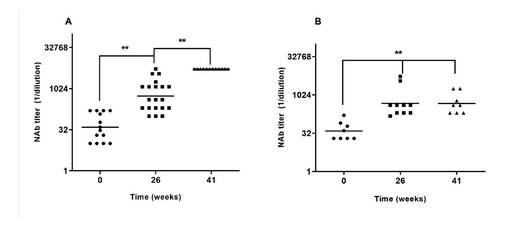
E2-CD154 is a subunit vaccine candidate that has been proven to be safe and to protect piglets from classical swine fever (CSF). In this study, those previous findings were confirmed and extended to a larger number of animals in a phase III clinical trial conducted on two production farms in Pinar del Río province. All animals in both farms were vaccinated with two doses of E2-CD154 on days 0 and 21. The study extended up to 60 weeks. The vaccine was well tolerated in piglets between 15 and 28 days of life, with neither local nor systemic side effects documented. Immunized pregnant sows were capable of transmitting high levels of maternally-derived neutralizing antibodies (MDNAs) to their offspring (Unit A, geometric mean titer = 1:1295, minimum value 1:100 and Unit B geometric mean titer = 1:474, minimum value 1:150), well above the protection threshold (1:50). These high MDNA titers in the piglets did not interfere with the immunogenicity of the candidate. All vaccinated piglets evaluated at random (more than 10% of 2804 vaccinated) developed protective neutralizing antibody titers higher than 1:400 at the four time points analyzed (nine, 21, 41, and 44 weeks) in both farms. The results of this study confirm the safety, immunogenicity and robustness of this vaccine candidate in this sensitive pig category in the field.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
Ganges L, Crooke HR, Bohórquez JA, Postel A, Sakoda Y, Becher P, et al. Classical swine fever virus: The past, present and future. Virus research. 2020;289:198151.
Lamothe-Reyes Y, Bohórquez JA, Wang M, Alberch M, Pérez-Simó M, Rosell R, et al. Early and Solid Protection Afforded by the Thiverval Vaccine Provides Novel Vaccination Alternatives Against Classical Swine Fever Virus. Vaccines. 2021;9(5):464.
Li F, Li B, Niu X, Chen W, Li Y, Wu K, et al. The Development of Classical Swine Fever Marker Vaccines in Recent Years. Vaccines. 2022;10(4):603.
Postel A, Austermann-Busch S, Petrov A, Moennig V, Becher P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transboundary and emerging diseases. 2018;65:248-61.
Coronado L, Perera CL, Rios L, Frías MT, Pérez LJ. A Critical Review about Different Vaccines against Classical Swine Fever Virus and Their Repercussions in Endemic Regions. Vaccines. 2021;9(2):154.
Bohórquez JA, Wang M, Díaz I, Alberch M, Pérez-Simó M, Rosell R, et al. The FlagT4G Vaccine Confers a Strong and Regulated Immunity and Early Virological Protection against Classical Swine Fever. Viruses. 2022;14(9):1954.
Choe S, Kim J-H, Kim K-S, Song S, Kang W-C, Kim H-J, et al. Impact of a live attenuated classical swine fever virus introduced to Jeju island, a CSF-free area. Pathogens. 2019;8(4):251.
Coronado L, Rios L, Frías MT, Amarán L, Naranjo P, Percedo MI, et al. Positive selection pressure on E2 protein of classical swine fever virus drives variations in virulence, pathogenesis and antigenicity: Implication for epidemiological surveillance in endemic areas. Transboundary and emerging diseases. 2019;66(6):2362-82.
Jang G, Kim JA, Kang WM, Yang HS, Park C, Jeong K, et al. Endemic outbreaks due to the re-emergence of classical swine fever after accidental introduction of modified live LOM vaccine on Jeju Island, South Korea. Transboundary and emerging diseases. 2019;66(2):634-9.
Sang HJ, Kwon T, Yoo SJ, Lee D-U, Lee S, Richt JA, et al. Classical swine fever outbreak after modified live LOM strain vaccination in naive pigs, South Korea. Emerging infectious diseases. 2018;24(4):798.
Suárez M, Sordo Y, Prieto Y, Rodríguez MP, Méndez L, Rodríguez EM, et al. A single dose of the novel chimeric subunit vaccine E2-CD154 confers early full protection against classical swine fever virus. Vaccine. 2017.
Sordo-Puga Y, Suárez-Pedroso M, Naranjo-Valdéz P, Pérez-Pérez D, Santana-Rodríguez E, Sardinas-Gonzalez T, et al. Porvac(r) Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus. Vaccines. 2021;9(2):167.
Muñoz-González S, Sordo Y, Pérez-Simó M, Suarez M, Canturri A, Rodriguez MP, et al. Efficacy of E2 glycoprotein fused to porcine CD154 as a novel chimeric subunit vaccine to prevent classical swine fever virus vertical transmission in pregnant sows. Veterinary microbiology. 2017;205:110-6.
Sordo-Puga Y, Pérez-Pérez D, Montero-Espinosa C, Oliva-Cárdenas A, Sosa-Teste I, Duarte CA, et al. Immunogenicity of E2CD154 Subunit Vaccine Candidate against Classical Swine Fever in Piglets with Different Levels of Maternally Derived Antibodies. Vaccines. 2021;9(1):7.
Oliva-Cárdenas A, Fernández-Zamora F, Santana-Rodríguez E, Sordo-Puga Y, Vargas-Hernández M, Rodríguez-Moltó M, et al. Safety and immunogenicity in piglets of two immunization schedules initiated at two or three weeks of age with PorvacÒ, a classical swine fever subunit marker vaccine. Bionatura. 2021;6(3).
Pérez-Pérez D, Sordo-Puga Y, Rodríguez-Moltó MP, Sardina T, Santana E, Montero C, et al. E2-CD154 vaccine candidate is safe and immunogenic in pregnant sows, and the maternal derived neutralizing antibodies protect piglets from classical swine fever virus challenge. Veterinary Microbiology. 2021:109153.
VICH. Target Animal Safety for Veterinary live and inactivated Vaccines, GL44. 2010.
Lopez O, editor. Manual De Procedimientos Te´cnicos Para La Crianza Porcina. La Habana, Cuba: Instituto de Investigaciones Porcinas : CIMA.; 2008.
Santana-Rodríguez E, Méndez-Orta M, Sardina-González T, Rodríguez-Moltó M, Castell-Brizuela S, Sordo-Puga Y, et al. Consistency of the Neutralizing Peroxidase Linked Assay for Classical Swine Fever and Homologation with an OIE Reference Laboratory. International Journal of Scientific Research in Biological Sciences. 2022;9(2):30-4.
Biront P, Leunen J, Vandeputte J. Inhibition of virus replication in the tonsils of pigs previously vaccinated with a Chinese strain vaccine and challenged oronasally with a virulent strain of classical swine fever virus. Veterinary microbiology. 1987;14(2):105-13.
Bouma A, de Smit AJ, de Kluijver EP, Terpstra C, Moormann RJ. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet Microbiol. 1999;66(2):101-14.
Terpstra C, Wensvoort G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol. 1988;16(2):123-8.
Parchariyanon S, Tantaswasdi U, Pinyochon W, Methiyapun P. Immunity against swine fever vaccine. II. Immunity against swine fever vaccine in piglets and protection level of maternal immunity in piglets before vaccination J Thai Vet Med Assoc. 1994;45(2):37-45.
Lipowski A, Drexler C, Pejsak Z. Safety and efficacy of a classical swine fever subunit vaccine in pregnant sows and their offspring. Veterinary microbiology. 2000;77(1-2):99-108.
Brun A, Barcena J, Blanco E, Borrego B, Dory D, Escribano JM, et al. Current strategies for subunit and genetic viral veterinary vaccine development. Virus Res. 2011;157(1):1-12.
Lim SI, Song JY, Kim J, Hyun BH, Kim HY, Cho IS, et al. Safety of classical swine fever virus vaccine strain LOM in pregnant sows and their offspring. Vaccine. 2016;34(17):2021-6.
Jackson PG, Cockcroft PD. Handbook of pig medicine: Elsevier Health Sciences; 2007.
Perri AM, O'Sullivan TL, Harding JC, Wood RD, Friendship RM. Hematology and biochemistry reference intervals for Ontario commercial nursing pigs close to the time of weaning. The Canadian Veterinary Journal. 2017;58(4):371.
Muirhead MR, Alexander TJ. Managing pig health and the treatment of disease: A reference for the farm: 5M Enterprises Ltd., PO Box 233.; 1997.
Reinoso Espin LV. Evaluación de la influencia del jugo de caña y un núcleo proteico en el perfil hepático en cerdos en etapa de crecimiento 2014.
Meyer DJ, Harvey JW. Veterinary Laboratory Medicine: Interpretation & Diagnosis: Saunders; 2004.
Chen J-Y, Wu C-M, Chen Z-W, Liao C-M, Deng M-C, Chia M-Y, et al. Evaluation of classical swine fever E2 (CSF-E2) subunit vaccine efficacy in the prevention of virus transmission and impact of maternal derived antibody interference in field farm applications. Porcine health management. 2021;7(1):1-14.