Effect of membrane protector and thawing solution on the in vitro quality of frozen goat semen in pellets
Main Article Content
Abstract
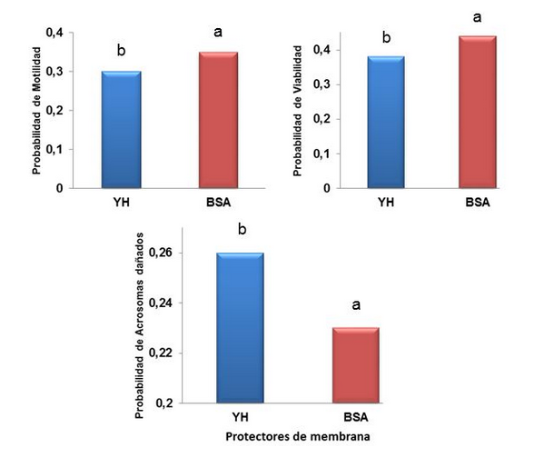
The aim of this research was to evaluate the effect of membrane protector and thawing solution on the in vitro quality of goat semen frozen in pellets. Two hundred and fourteen ejaculates, collected twice a week by artificial vagina, were processed. The parameters volume, motility, concentration, viability, and total sperm abnormalities were evaluated. Suitable ejaculates were mixed and divided into two portions. Each received the corresponding formulation without carrying out the sperm washing by centrifugation. A non-commercial freeze-dried diluent composed of Tris-Glucose-Citric Acid and Glycerol, with different membrane protectors, egg yolk (4.45%) or BSA (5%), was used. It was frozen in vapor phase nitrogen (0.1 mL pellets), stored in liquid nitrogen for seven days. For thawing, two solutions (Tris and CIMATE) and a control (dry tube without thawing solution) were used. Percent motility (30, 120 and 240 minutes), viability and damaged acrosomes (30 and 120 minutes) were determined in incubation test. Membrane protector, thawing solution and their interactions were compared using a Binary Logistic Regression model. The combination of BSA and CIMATE presented the highest probability (P<0.05) of motility and viability at all times and the lowest probability (P<0.05) of damaged acrosomes at 30 min and 120 min. It is concluded that BSA can be used as a membrane protector for goat semen frozen in pellets in a freeze-dried Tris-based diluent, without carrying out sperm washing by centrifugation. In addition, CIMATO solution, as a thawing agent, enables better sperm cell recovery.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
Grötter LG, Cattaneo L, Marini PE, Kjelland ME, Ferré LB. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post‐thaw quality optimization. Reprod Domest. An. 2019;54(4):655-665. https://doi.org/10.1111/rda.13409
Ntemka A, Tsakmakidis IA, Kiossis E, Milovanović A, Boscos CM. Current status and advances in ram semen cryopreservation. J Hellenic Vet Med Soc. 2018;69(2):911-924. https://ejournals.epublishing.ekt.gr/index.php/jhvms/article/view/18014
Peris-Frau P, Soler AJ, Iniesta-Cuerda M, Martín-Maestro A, Sánchez-Ajofrín I, Medina-Chávez DA, et al. Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020;21(8):2781. https://doi.org/10.3390/ijms21082781
Sun L, Fan W, Wu C, Zhang S, Dai J, Zhang D. Effect of substituting different concentrations of soybean lecithin and egg yolk in tris-ased extender on goat semen cryopreservation. Cryobiology. 2020;92:146-150. DOI:10.1016/j.cryobiol.2019.12.004
Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction, 2020;159(4):189-201. https://doi.org/10.1530/REP-19-0452
Kumar A, Prasad JK, Srivastava N, Ghosh SK. Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreservation and Biobanking. 2019;17(6):603-612. http://doi.org/10.1089/bio.2019.0037
Karunakaran M, Devanathan TG. Evaluation of bull semen for fertilityassociated protein, in vitro characters and fertility. J Appl Anim Res. 2017;45:136-144. DOI:10.1080/09712119.2015.1129343.
Santiago-Moreno J, Galarza DA. Sperm cryopreservation in domestic and wild species: a review of recent advances. Rev Ec Ciencia An. 2019;3(2):18-38. ISSN 2602-8220.
Zamiri MJ. Update on semen cryopreservation in sheep and goats: A review. Journal of livestock science and technologies. 2020;8(1):1-15. DOI:10.22103/jlst.2020.15927.1321.
Khan IM, Cao Z, Liu H, Khan A, Rahman SU, Khan MZ, et al. Impact of Cryopreservation on Spermatozoa Freeze-Thawed Traits and Relevance OMICS to Assess Sperm Cryo-Tolerance in Farm Animals. Front. Vet. Sci. 2021;8:609180. DOI: 10.3389/fvets.2021.609180
Sharma A, Sood P. Cryopreservation and fertility of frozen thawed Chegu goat semen. Indian J. Anim. Res. 2019; 53(11): 1414-1419. DOI:10.18805/ijar.B-3696
Martínez-Durán J, Duverger-Tellez O, Díaz-Martínez N, Interian-Alvarez L, Denis-García R, Palacios-Espinosa A. Effect of the sperm membrane protector on the freezability of goat semen. Tropical and Subtropical Agroecosystems. 2022;25(2). https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/4011
Sandal AI, Senlikci H, Baran A, Ozdas OB. Effects of semen extender supplemented with Bovine Serum Albumin (BSA) on spermatological traits of Saanen buck semen stored at +4°C. Kafkas Univ Vet Fak Derg. 2020;26(4):515-520. DOI: 10.9775/kvfd.2019.23674.
Sharma A, Sood P. Caprine semen cryopreservation and the factors affecting it: An overview. Veterinary Sciences: Research and Reviews. 2020;6(1):46-57. http://dx.doi.org/10.17582/journal.vsrr/2020/6.1.46.57
Satorre MM, Breininger E. Effect of packaging method on quality and functional parameters in cryopreserved porcine spermatozoa with alpha tocopherol. Research in Veterinary Science and Medicin. 2021;1(1):1-6. DOI:10.25259/RVSM_4_2020.
Khalifa TAA, El-Saidy BE. Pellet-freezing of Damascus goat semen in a chemically defined extender. An Reprod Sci. 2006;93:303-315. DOI: 10.1016/j.anireprosci.2005.08.008
Minitab 19. Software estadístico, State College, Pensilvania: Minitab, Inc. 2019. www.minitab.com
- Agossou DJ, Koluman N. An Objective Analysis of Factors Affecting Buck Semen Quality Attributes during Cryopreservation:A Mini Review. Annual Research & Review in Biology, 2018;27(3):1-7. DOI: 10.9734/ARRB/2018/42087
Raheja N, Choudhary S, Grewal S, Sharma N, Kumar N. A review on semen extenders and additives used in cattle and buffalo bull semen preservation. J. Entomol. Zool. Stud. 2018;6(3):239-245.
Gangwar C, Kharche SD, Kumar S, Jindal SK. Cryopreservation of goat semen: status and prospects. Indian Journal of Small Rum. 2016;22(1):1-10. DOI: 10.5958/0973-9718.2016.00005.2
Silva ECB, Lima RA, Guerra MMP. Goat semen freezing: the two faces of the coin. An. Sci. 2021;31(1):134-144. http://www.uece.br/cienciaanimal/index.php?option=com_docman&task=doc_view&gid=797&tmpl=component&format=raw&Itemid=157
Mahdi SAAW, Mahmood FA, Mahmoo RM. Effect of different concentrations of Bovine Serum Albumin on some of the frozen sperm characteristics of the rams. Plant Archives. 2019;19(2):1486-488.
Alcay S, Toker MB, Gokce E, Onder NT, Ustuner B, Nur Z. Long term incubation resilience of post-thaw ram semen diluted with lecithin-based extender supplemented with Bovine Serum Albumin. Kafkas Univ Vet Fak Derg. 2019;25(3):291-297. https://doi.org/10.9775/kvfd.2018.20843
Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet Brno. 2007;76:383-390. https://doi.org/10.2754/avb200776030383
Bustani GS, Baiee FH. Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders, Veterinary World. 2021;14(5):1220-1233. doi: doi.org/10.14202/vetworld.2021.1220-1233
Isachenko V, Sanchez R, Rahimi G, Mallmann P, Isachenko E, Merzenich M. Cryoprotectant-free vitrification of spermatozoa: Fish as a model of human. Andrologia. 2019;51(1):e13166. https://doi.org/10.1111/and.13166
Fhulufhelo RV, Khoboso L, Tshimangadzo N. Cryopreservation of South African Indigenous Goat Semen. Alemania. Ed. Lambert Academic Publishing (LAP). 2012. ISBN 10: 3848442469 / ISBN 13: 9783848442461.
Evans G, Maxwell WMC. Frozen storage of semen. In: Salamon's Artificial Insemination of Sheep and Goats. Butterworths, Wellington. 1987;122-141. ISBN: 9780409491777
Yamashiro H, Wang H, Yamashita Y, Kumamoto K, Terada T. Enhanced Freezability of Goat Spermatozoa Collected into Tubes Containing Extender Supplemented with Bovine Serum Albumin (BSA). J. Reprod. Dev. 2006;52(3):407-414. DOI:10.1262/jrd.17105.
Martínez J, Interián L, Valdés M, Milanés C, Collazo J. Influencia del protector de la membrana espermática, el crioprotector y la solución descongelante en la congelabilidad del semen caprino. Rev. Cub. Reprod. Anim. 2004;30(1-2):81-89.
Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, et al. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37(3):327-339. DOI: 10.1016/j.rbmo.2018.05.012
Viotty G. Procesamiento del semen bovino para la Inseminación Artificial. Tesis Doctoral. Univ. Montevideo, Fac. Vet., Uruguay. 2012.
Duverger O, Moya A, Barba FJ, Hernández JJ. Nuevos diluyentes y descongelantes para semen bovino. Rev. Cub. Cient. Vet. 1988;19(1):19-28.
Awad MM, Graham JK. A new pellet technique for cryopreserving ram and bull spermatozoa using the cold surface of cattle fat. Animal Reproduction Science. 2004;84:83-92. https://doi.org/10.1016/j.anireprosci.2003.12.001
Moraes C, Neves J, Goncalves P, Oliveira J, Schweitzer C. Criopreservacao do semen ovino en pellets. Ciencia Rural. 1998;28(2):287-292. https://doi.org/10.1590/S0103-84781998000200018
Saadeldin IM, Khalil WA, Alharbi MG, Lee SH. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals. 2020;10(12):2281. DOI:10.3390/ani10122281