Vitrification of pre-implantation embryos a simple method for the preservation and production of transgenic mice
Main Article Content
Abstract
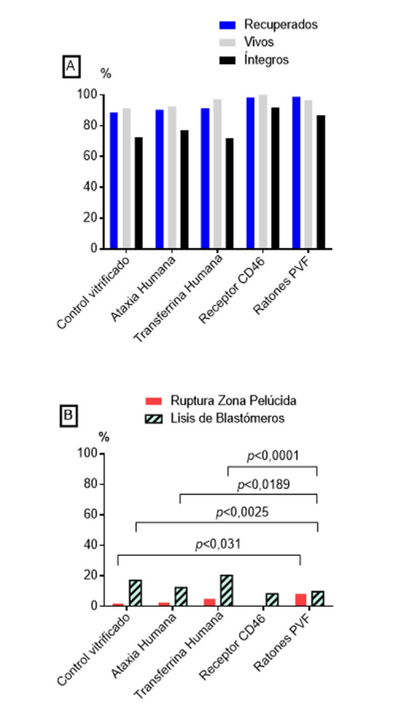
Vitrification of embryos and gametes is a novel alternative for ex situ preservation and production of transgenic mice. The first objective of this research was to demonstrate the effectiveness of vitrifying 4-cell embryos for the preservation of genetic lines from four transgenic mouse models: 1) Ataxia, 2) Human transferrin, 3) CD46 receptor, and 4) transgenic mice expressing green fluorescent protein (GFP). A second objective was to produce transgenic offspring from vitrified pronuclear embryos that had been genetically modified via lentiviral transgenesis. The embryos obtained from non-transgenic mice served as the control group. Those embryos at the 4-cell and pronuclear stages were collected 55 hours and 18 hours, respectively, after superovulatory treatment. The embryos collected were vitrified using specific mixtures of DMSO, ethylene glycol and sucrose; and they were subsequently stored in liquid nitrogen until needed. In vitro and in vivo survival was evaluated in each experimental group, as well as the principal cytostructural damage suffered by the embryos after thawing. In the first experiment, a high embryonic survival rate was reached in all four transgenic lines after the vitrification of embryos at the 4-cell stage, ranging from 91,5 % to 100 %. In the case of in vivo development, no significant differences were observed in the percentages of viable offspring born among the transgenic lines Ataxia (49,5 %), Transferrin (47 %) and CD46 receptor (24,4 %), compared to the vitrified control group (38,3 %) (p>0,05). The main cytostructural damages observed were the rupture of the zona pellucida and lysis of blastomeres, with percentages differing among groups. In the case of pronuclear embryos, a 19,9 % viable offspring rate was obtained (n=52 live births/266 embryos transferred). Of these, 25 % (n=13/52) of the offspring obtained were transgenic, resulting in an overall efficiency of 4,5 % (n=13/266) for lentiviral transgenesis using vitrified pronuclear embryos. The results obtained demonstrate that embryo vitrification is an effective method for preserving mouse lines and producing transgenic mice through lentiviral transgenesis.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
Bolton R.L., Mooney A., Pettit M.T., Bolton A.E., Morgan L., Drake G.J., et al. Resurrecting biodiversity: advanced assisted reproductive technologies and biobanking. Reprod Fertil. 2022;3(3):R121-R46.
Holt W.V., Comizzoli P. Genome resource banking for wildlife conservation: promises and caveats. Cryo Letters. 2021;42(6):309-20.
Leibo S.P., Sztein J.M. Cryopreservation of mammalian embryos: Derivation of a method. Cryobiology. 2019;86:1-9.
Awasthi P.R., French C.F., Sztein J., Bedigian R., Sharp J.J., Lloyd K.C. Frozen sperm as an alternative to shipping live mice. Contemp Top Lab Anim Sci. 2003;42(5):8-11.
Agca Y., Agca C. Cryopreservation and Transplantation of Laboratory Rodent Ovarian Tissue for Genome Banking and Biomedical Research. Methods Mol Biol. 2021;2180:469-83.
Takeo T., Nakao S., Nakagawa Y., Sztein J.M., Nakagata N. Cryopreservation of mouse resources. Lab Anim Res. 2020;36:33.
Shaw J.M., Nakagata N. Cryopreservation of transgenic mouse lines. Methods Mol Biol. 2002;180:207-28.
Hart-Johnson S., Mankelow K. Archiving genetically altered animals: a review of cryopreservation and recovery methods for genome edited animals. Lab Anim. 2022;56(1):26-34.
Boubelik M., Cerna Z. A modified two-step method for cryopreservation of mouse embryos for purposes of embryo banking. Folia Biol (Praha). 1993;39(4):211-9.
Taft R. Mouse Embryo Cryopreservation by Slow Freezing. Cold Spring Harb Protoc. 2018;2018(5).
Shaw J.M., Diotallevi L., Trounson A.O. A simple rapid 4.5 M dimethyl-sulfoxide freezing technique for the cryopreservation of one-cell to blastocyst stage preimplantation mouse embryos. Reprod Fertil Dev. 1991;3(5):621-6.
Trounson A., Peura A., Kirby C. Ultrarapid freezing: a new low-cost and effective method of embryo cryopreservation. Fertil Steril. 1987;48(5):843-50.
Oikonomou Z, Chatzimeletiou K, Sioga A, Oikonomou L, Tarlatzis B.C., Kolibianakis E. Effects of vitrification on blastomere viability and cytoskeletal integrity in mouse embryos. Zygote. 2017;25(1):75-84.
Ghandy N, Karimpur Malekshah A.A. Which Stage of Mouse Embryos Is More Appropriate for Vitrification? Int J Fertil Steril. 2017;10(4):357-62.
Shaw J.M., Jones G.M. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9(6):583-605.
Vajta G. Vitrification of the oocytes and embryos of domestic animals. Anim Reprod Sci. 2000;60-61:357-64.
Ali J, Shelton J.N. Vitrification of preimplantation stages of mouse embryos. J Reprod Fertil. 1993;98(2):459-65.
Nakao K, Nakagata N, Katsuki M. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp Anim. 1997;46(3):231-4.
Zhou G.B., Hou Y.P., Jin F, Yang Q.E., Yang Z.Q., Quan G.B., et al. Vitrification of mouse embryos at various stages by open-pulled straw (OPS) method. Anim Biotechnol. 2005;16(2):153-63.
Zhang J, Cui J, Ling X, Li X, Peng Y, Guo X, et al. Vitrification of mouse embryos at 2-cell, 4-cell and 8-cell stages by cryotop method. J Assist Reprod Genet. 2009;26(11-12):621-8.
Kaneko T, Nakagawa Y. Genome editing of rodents by electroporation of CRISPR/Cas9 into frozen-warmed pronuclear-stage embryos. Cryobiology. 2020;92:231-4.
Keskintepe L, Agca Y, Pacholczyk G.A., Machnicka A, Critser J.K. Use of cryopreserved pronuclear embryos for the production of transgenic mice. Biol Reprod. 2001;65(2):407-11.
Bagis H, Odaman H, Sagirkaya H, Dinnyes A. Production of transgenic mice from vitrified pronuclear-stage embryos. Mol Reprod Dev. 2002;61(2):173-9.
Maemura M, Taketsuru H, Nakajima Y, Shao R, Kakihara A, Nogami J, et al. Totipotency of mouse zygotes extends to single blastomeres of embryos at the four-cell stage. Sci Rep. 2021;11(1):11167.
Markoulaki S, Meissner A, Jaenisch R. Somatic cell nuclear transfer and derivation of embryonic stem cells in the mouse. Methods. 2008;45(2):101-14.
Sung LY, Chang CC, Amano T, Lin CJ, Amano M, Treaster SB, et al. Efficient derivation of embryonic stem cells from nuclear transfer and parthenogenetic embryos derived from cryopreserved oocytes. Cell Reprogram. 2010;12(2):203-11.
Montoliu L. Transgenesis and Genome Engineering: A Historical Review. Methods Mol Biol. 2023;2631:1-32.
Aguiar J, Fernandez J, Aguilar A, Mendoza Y, Vazquez M, Suarez J, et al. Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specific Purkinje cell degeneration in transgenic mice. Neurosci Lett. 2006;392(3):202-6.
Perera Y, Cobas K, Garrido Y, Nazabal C, Brown E, Pajon R. Determination of human transferrin concentrations in mouse models of neisserial infection. J Immunol Methods. 2006;311(1-2):153-63.
Riego E, Limonta J, Aguilar A, Perez A, de Armas R, Solano R, et al. Production of transgenic mice and rabbits that carry and express the human tissue plasminogen activator cDNA under the control of a bovine alpha S1 casein promoter. Theriogenology. 1993;39(5):1173-85.
Toledo JR, Prieto Y, Oramas N, Sanchez O. Polyethylenimine-based transfection method as a simple and effective way to produce recombinant lentiviral vectors. Appl Biochem Biotechnol. 2009;157(3):538-44.
Zhao XM, Quan GB, Zhou GB, Hou YP, Zhu SE. Conventional freezing, straw, and open-pulled straw vitrification of mouse two pronuclear (2-PN) stage embryos. Anim Biotechnol. 2007;18(3):203-12.
Takeo T, Nakagata N. Cryobanking and Recovery of Genetically Modified Mice. Methods Mol Biol. 2020;2066:195-209.
Behringer R, Gertsenstein M, Nagy KV, Nagy A. Administration of Gonadotropins for Superovulation in Mice. Cold Spring Harb Protoc. 2018;2018(1).
Lamas S, Carvalheira J, Gartner F, Amorim I. C57BL/6J mouse superovulation: schedule and age optimization to increase oocyte yield and reduce animal use. Zygote. 2021;29(3):199-203.
Luo C, Zuniga J, Edison E, Palla S, Dong W, Parker-Thornburg J. Superovulation strategies for 6 commonly used mouse strains. J Am Assoc Lab Anim Sci. 2011;50(4):471-8.
Hasegawa A, Mochida K, Inoue H, Noda Y, Endo T, Watanabe G, et al. High-Yield Superovulation in Adult Mice by Anti-Inhibin Serum Treatment Combined with Estrous Cycle Synchronization. Biol Reprod. 2016;94(1):21.
Pu XA, Young AP, Kubisch HM. Production of Transgenic Mice by Pronuclear Microinjection. Methods Mol Biol. 2019;1874:17-41.
Cheng PH, Chang YF, Mao SH, Lin HL, Chen CM, Yang SH. Lentiviral transgenesis in mice via a simple method of viral concentration. Theriogenology. 2016;86(6):1427-35.
Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868-72.
Pfeifer A, Hofmann A. Lentiviral transgenesis. Methods Mol Biol. 2009;530:391-405.
Miao K, Guo M, An L, Xu XL, Wu H, Wang D, et al. A new method to efficiently produce transgenic embryos and mice from low-titer lentiviral vectors. Transgenic Res. 2011;20(2):357-63.
Koza P, Przybys J, Klejman A, Olech-Kochanczyk G, Konopka W. Generation of Transgenic Rats using a Lentiviral Vector Approach. J Vis Exp. 2020(159).