Expanded acute toxicity by intranasal administration of SURFACEN® in rats
Main Article Content
Abstract
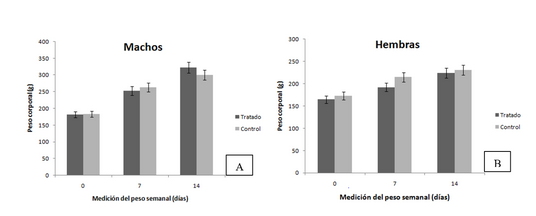
Local irritability studies for inhaled products constitute a fundamental link in the chain of regulatory requirements for preclinical toxicological studies. For this reason, the objective of the work was to determine the irritation potential of SURFACEN® in repeated doses by intranasal route. Thirty Cenp: Sprague Dawley rats (15/sex) were divided into two experimental groups: control (0.9 % NaCl solution) and treated (SURFACEN®). Both groups received the same treatment scheme with two daily intranasal applications spaced four to six hours apart, for 14 days. The dose to be applied was 13.27 mg/kg. Clinical observations, body weight, post mortem body weight, absolute and relative weight of the selected organs (lungs, brain) of each animal and pathological study were the variables to be studied. The design and implementation of the research was carried out according to the animal welfare principles. All animals survived the study. No statistically significant differences were evidenced between both groups regarding the variables studied. There were no signs of overt toxicity after SURFACEN® administration, and no anatomopathological alterations that could be associated with the administration of the drug were detected. It was evidenced that the application of repeated doses of SURFACEN® by intranasal route did not alter the cell morphology of the nasal mucosa, did not cause histopathological damage in the organs studied and did not produce signs of local irritation, thus it is considered potentially non-toxic for humans.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
García Cases S, Caro Aragonés I, Aguinagal de Toya A, Gaspar Carreño M, Marquez Peiró JF. Dispositivos y guía de administración vía inhalatoria. ILAPHAR Rev. OFIL. 2017;27(1):31-46. Disponible en: https://www.ilaphar.org/dispositivos-guia-administracion-via-inhalatoria/
Bautista-Méndez R, Salinas-Lezama E, Bonola-Gallardo LJ, Pineda-Gudiño RD, Magdaleno-Maldonado GE. Selección en la diversidad de inhaladores: una actualización de bolsillo. Neumol Cir Torax. 2020;79(3):204-207. Disponible en: https://dx.doi.org/10.35366/96657
Anderson S, Atkins P, Beackman P, Cipolla D, Clark A, Daviskas E, et al. Inhaled Medicines: Past, Present, and Future. Pharmacol Rev. 2022;74(1):48-118. Disponible en: https://doi.org/10.1124/pharmrev.120.000108
Díaz-Casañas E, Morilla Gúzmán A, Rodríguez Moya V, Barrese Pérez Y. Estrategia de desarrollo clínico para la evaluación de la eficacia y seguridad de SURFACEN®. Rev Arch Med Camagüey. 2019;23(4):455-463. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-02552019000400455
Díaz-Casañas E, Rodríguez Moya V, Montes de Oca Martínez N. Surfactante pulmonar: posible intervención frente al nuevo Síndrome Respiratorio Agudo Severo Coronavirus 2 (SARS-CoV-2). Rev Habanera Cienc Médi. 2020;19:1-5. Disponible en: http://www.revhabanera.sld.cu/index.php/rhab/article/view/3361
Blanco O, Ramírez W, Lugones Y, Díaz E, Morejón A, Rodríguez V, et al. Protective effects of Surfacen® in allergen-induced asthma mice model. Int Immunopharmacol. 2022;102. Disponible en: https://doi.org/10.1016/j.intimp.2021.108391
Comisión Europea. Anexo 5 Parte B: Métodos para la determinación de la toxicidad y otros efectos sobre la salud. The Directive on Dangerous Substances. 2019. Disponible en: https://ec.europa.eu/environment/archives/dansub/pdfs/annex5b_es.pdf
Willson DF. Aerosolized Surfactants, Anti-Inflammatory Drugs, and Analgesics. Respir Care. 2015;60(6):774-790; Disponible en: https://doi.org/10.4187/respcare.03579
Center for Drug Evaluation and Research (CDER). Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Pharmacol Toxicol. July 2005. Disponible en: https://www.semanticscholar.org/paper/Guidance-for-Industry-Estimating-the-Maximum-Safe-Alert/8750b5d4c6564d44ac84826e3760f15ea0b836de
Derelanko MJ, Auletta CS. Handbook of Toxicology. Third Edition 2014 ISBN 9781439890134. Published March 7, 2014 by CRC Press 1024 Pages 7 Color & 340 B/W Illustrations.
European Agency for the Evaluation of Medicinal Products. Guideline on non-clinical local tolerance testing of medicinal products. EMEA/ CHMP/SWP/2145/2000. 1-9. 2015, London.
Guías para la eutanasia de los animales 2020 AVMA. https://www.portalveterinaria.com/upload/202002070922022020_Euthanasia_Final_1-15-20.pdf
Aller Reyero MA, Rodríguez Gómez J, Rodríguez Fabiána G. Normas éticas para el cuidado y utilización de los animales de experimentación. Rev Cirugía Esp. 2000;67(1):10-13. https://www.elsevier.es/es-revista-cirugia-espanola-36-articulo-normas-eticas-el-cuidado-utilizacion-8848
Morilla GA, Díaz-Casañas E, Ávila AY, Barrese PY, Fernández LO, Uranga PR. Seguridad del tratamiento con Surfacen® en recién nacidos pretérminos con síndrome de dificultad respiratoria. Rev Cubana Pediatr. 2019;91(2):1-14. Disponible en: http://www.revpediatria.sld.cu/index.php/ped/article/view/700/315
Barrese PY, Lim AN, Díaz-Casañas E, Uranga PR, Ávila AY. Evaluación de la seguridad del uso poscomercial del surfactante cubano, SURFACEN®, en el tratamiento del síndrome de dificultad respiratoria aguda del adulto. Neumol Cir Torax. 2019;78(3):296-303. DOI:10.35366/NT193F
Rodríguez-Moya VS, Barrese-Pérez Y, Uranga-Piña R, Díaz-Pérez L, Verdecia-Sánchez L, Díaz-Casañas E. Seguridad del tratamiento con surfactante pulmonar en el síndrome de dificultad respiratoria aguda en niños. Rev haban cienc méd 2022; 21(1). http://www.revhabanera.sld.cu/index.php/rhab/article/view/4362
Alemán C. Reference data base for the principal physiological indicators in species of laboratory animal. Lab Anim. 2000;34(1):358-378. DOI:10.1258/002367700780387741
Taconic Technical Library. [Homepage on the internet]. Cambridge: Taconic Sprague Dawley, Growth and Phenotyping Data; 2018. http://www.taconic.com/rat-model/sprague-dawley
Rosario Fernández LA, Tamargo Santos B, Batista Santiesteban N, Infante Bourzac JF, Sierra GonzálesVG, Arencibia Arrebola DF, et al. Estudio de tolerancia local de un candidato vacunal proteoliposómico contra Leptospira spp en el biomodelo Mesocricetus auratus. Theoria. 2012; 21(1):21-36. Disponible en: https://www.redalyc.org/articulo.oa?id=29931769003
Oliva-Hernández R, Fariñas-Medina M, Infante-Bourzac JF, Hernández-Salazar T, Núñez-Martínez D, Quintero-Pérez A, et al. Estudio de tolerancia local de la vacuna antimeningocóccica VA-MENGOC-BC® en ratas Sprague Dawley. Evaluación a los 24 y 36 meses en estante VacciMonitor. 2019;28(1):9-18. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-028X2019000100009&lng=es
Paixao A, Mancebo B, Regalado AI, Chong D, Sánchez LM. Evaluación de la Toxicidad Aguda Oral del extracto etanólico de Tephrosia vogelii Hook (kalembe). Rev Salud Animal. 2017; 39(2). http://revistas.censa.edu.cu/index.php/RSA/article/view/895/876
Infante JF, Sifontes S, Pérez V, Bracho G, Hernández T, Zayas C, et al. Ensayo de inmunogenicidad y toxicidad local del cocleato de Neisseria meningitidis en ratas Sprague Dawley. VacciMonitor. 2009;188(1):1-7. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-028X2009000100001
Greaves P. Histopathology of Preclinical Toxicity Studies. Interpretation and Relevance in Drug Safety Evaluation. 4th Edition - October 7, 2011, Elsevier. United Kingdom. eBook ISBN: 9780444538611. Hardcover ISBN: 9780444538567. Disponible en: https://www.elsevier.com/books/histopathology-of-preclinical-toxicity-studies/greaves/978-0-444-53856-7
Draft OECD Guidance Document on Histopathology for inhalation toxicity studies, Supporting TG412 (Subacute Inhalation Toxicity: 28-Day) and TG 413 (Subchronic Inhalation Toxicity: 90-Day). Version 28, September 2009. Disponible en: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-gd125.pdf
Oros Pantoja R. Efecto del estrés sobre el tejido linfoide asociado a nariz en el ratón. 2011. http://www.repositoriodigital.ipn.mx/handle/123456789/12086
Takaki H, Ichimiya S, Matsumoto M, Seya T. Mucosal Immune Response in Nasal- Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J Innate Immun. 2018;10:515-52. DOI: 10.1159/000489405