Implementation in Cuba of a molecular assay for the detection of Brucella spp. in humans
Main Article Content
Abstract
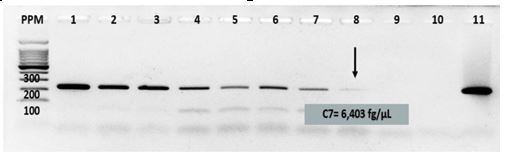
Brucellosis is a zoonosis that may cause mild symptoms or severe manifestations. Molecular techniques are very sensitive and specific in the timely and early detection of Brucella spp. DNA, both at the beginning of the infection and in relapses and therapeutic failures. The aim of the present research was to implement a conventional PCR-bcsp that would allow the timely diagnosis of human brucellosis in Cuba, and thus contribute to avoid the occurrence of complications that can lead to disabling sequelae in the patient. A service and system research was carried out at the National Reference Laboratory for Leptospira and Brucella at IPK to evaluate the PCR-bcsp. The parameters of analytical sensitivity and specificity, as well as diagnostic sensitivity and specificity were determined. Conventional PCR was applied to 86 sera from suspected cases of human brucellosis that were negative to the IgM and IgG ELISAs for Brucella. PCR-bcsp presented a detection limit of 6.403 fg/µL. Analytical specificity was 100 %, while the diagnostic sensitivity and specificity showed values of 96.3 % and 100 %, respectively. After its application to sera from serologically negative probable cases of human brucellosis, 85 % positivity was reached. The present research strengthened the microbiological diagnosis of human brucellosis since a molecular tool guaranteeing the early diagnosis of the disease in Cuban environment has been implemented.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
Laine CG, Johnson VE, Scott HM, ArenasGamboa AM. Global Estimate of Human Brucellosis Incidence. Emerg Infect Dis. 2023; 29(9):1789-97. DOI: 10.3201/eid2909.23 0052. PMID: 37610167; PMCID: PMC1046 1652.
Alharbi MGT, Alanazi AS, Alanazi NF, Alsaleh AK, Alanazl SJ, Alanazi SM, et al. Overview of Brucellosis: Simple Review Article. Pharmacophore. 2022;13(2):101-6. Available in: https://doi.org/10.51847/oSqu90fp0k.
O'Callaghan D. Brucelosis humana: avances recientes y retos futuros.InfectDisPoverty. 2020; 9(101). Available in: https://idpjournal.biomed central.com/articles/10.1186/s40249-020-00715-1
Amjadi O, Rafiei A, Mardani M, Zafari P, Zarifian A. A review of the immunopathogenesis of Brucellosis. Infect Dis. 2019; 51 (5): 321-33. DOI: 10.1080/23744235.2019.1568545
Yagupsky P, Morata P, Colmenero JD. Laboratory Diagnosis of Human Brucellosis. ClinMicrobiol Rev. 2019;33(1):e00073-19. DOI: 10.1128/CMR.00073-19.
Thakura S, Bedia J, Singha R, Gillb K, Arorac A, Kashyap N. Quantitative polymerase chain reaction based quantification of Brucella DNA in serum of pre- and post-therapeutic occupationally exposed infected human population. Journal of Infection and Public Health 2018;11(4): 514-20. DOI:10.1016/j.jiph. 2017.10.004
Projahn M, Hammerl JA, Dieckmann R, Dahouk SA. A Proof of Principle for the Detection of Viable Brucella spp. in Raw Milk by qPCR Targeting Bacteriophages. Microorganisms. 2020;8(9):1326. DOI: 10.3390/microorganisms 8091326.
Wang Y, Wang Z, Zhang Y, Bai L, Zhao Y, Liu C, et al.. Polymerase chain reaction-based assays for the diagnosis of human brucellosis. Ann Clin Microbiol Antimicrob2014; 13:31. DOI: 10.118 6/s12941-014-0031-7
Lugo O, Obregón AM, Echevarría E, Rodríguez Y, Soto Y. Aspectos clínicos y epidemiológicos de la brucelosis humana en tres provincias cubanas (2013-2016). Rev Cubana Med Trop2022; 74(2):e784. Disponible en: https:// revmedtropical.sld.cu/index.php/medtropical/arti cle/view/784.
Baily GG, Krahn JB, Drasar BS, Stoker NG. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J. Trop. Med. Hyg. 1992; 95:271-5. PMID: 1495123.
Mukherjee F, Nagmani K, Surendra KSNL, Subramanian BM, Bahekar VS, Prasad A, et al.. Optimization and validation of a diagnostic realtime PCR for bovine brucellosis. AdvAnim VetSci. 2015; 3(11): 577-87. DOI:10.14737/ journal.aavs/2015/3.11.577.587.
QIAGEN. Manual de instrucciones de uso de QIAsymphony DSP DNA 2015; 40 p. Disponible en: http://www.qiagen.com.
Al- Khater B, Al- Ouqaili M, Al- Anii S. In Term of Molecular Technique, Taxonomic and Diagnostic Aspects of Chronic Human Brucellosis in Ramadi City. Anb Med J. 2020; 12(1):1-12. DOI: 10.33091/AMJ.0101212015.
Talmaci R, Traeger-Synodinos J, Kanavakis E, Coriu D, Colita D, Gavrila L. Scanning of betaglobin gene for identification of beta-thalassemia mutation in Romanian population. J Cell Mol Med. 2004;8(2):232-40. DOI:10.1111/j.1582-49 34.2004.tb00278.x.
Ariza X. Brucelosis. En: Rozman C, Cardellach F, Agustí A, et al., editores. Tratado de Medicina Interna Farreras-Rozman. Vol 2. 19ª ed. España: Elsevier; 2020. p. 2045-7.
Mitka S, Anetakis C, Souliou E, Diza E, Kansouzidou A. Evaluation of different PCR assays for early detection of acute and relapsing brucellosis in humans in comparison with conventional methods. J Clin Microbiol. 2007; 45(4):1211-8. DOI:10.1128/JCM.00010-06
Cecmed. Regulación No. 47-2007: Requisitos para la evaluación del desempeño de los diagnosticadores. Disponibleen: http://www.cecmed.cu/Pages/AmbReg-8.htm.
Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. Serum Is the Preferred Clinical Specimen for Diagnosis of Human Brucellosis by PCR. J. Clin. Microbiol. 2001; 39 (4): 16614. DOI:10.1128/JCM.39.4.1661-1664.2001.
Murray PR, Rosenthal KS, Pfaller MA. Brucella y Francisella. En: Murray PR, Rosenthal KS, Pfaller MA. Microbiologíamédica. 9na ed. España: Elsevier; 2021. p. 383-90.