Detection of multidrug-resistant Streptococcus equi subsp. zooepidemicus in Cuba
Main Article Content
Abstract
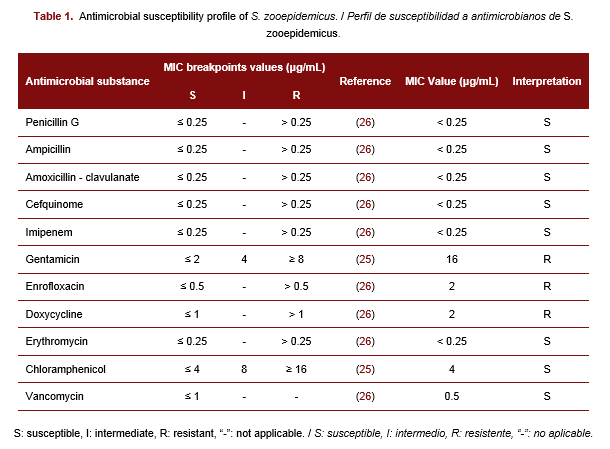
Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) is an opportunistic pathogen capable of causing various infections in the respiratory, genital, and urinary tracts of horses and other animals. This species is considered responsible for many emerging zoonotic diseases. There has been an increasing circulation of multidrug-resistant S. zooepidemicus strains in horses, however, there is no information on S. zooepidemicus and its antimicrobial susceptibility profile in Cuban horses. Therefore, the objectives of this study were to report the isolation of S. zooepidemicus in a horse and to determine its antimicrobial susceptibility profile. A mare with pale mucous membranes, owned by a private producer in Melena del Sur, Mayabeque, was sampled, and a swab was taken from the genital tract. The isolate obtained was identified using the analytical profile index and mass spectrometry. The minimal inhibitory concentration (MIC) of 11 antibiotics (penicillin G, ampicillin, amoxicillin-clavulanate, cefquinome, imipenem, gentamicin, enrofloxacin, doxycycline, erythromycin, chloramphenicol, and vancomycin) was determined for the isolate. The isolate was identified as S. zooepidemicus and it was susceptible to all β-lactam, erythromycin, chloramphenicol, and vancomycin. The isolate presented a multidrug resistance profile to gentamicin, enrofloxacin, and doxycycline with MIC values of 16 μg/mL, 2 μg/mL, and 2 μg/mL, respectively. For the first time in Cuba, multidrug-resistant S. zooepidemicus was detected in the genital mucosa of a mare. The close interaction between humans and horses increases the risk of acquiring these multiresistant microorganisms or favoring their dissemination, thus this result should be considered in staff training.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
De Vos P. e. Bergey´s manual of systematic bacteriology. The Firmicutes. 2nd ed. New York, USA: Springer; 2009. 1450 p.
Li J, Zhao Y, Gao Y, Zhu Y, Holyoak GR, Zeng S. Treatments for endometritis in mares caused by Streptococcus equi subspecies zooepidemicus: A structured literature review. J Equine Vet Sci. 2021;102:1-10.
Day MJ, Carey S, Clercx C, Kohn B, MarsilIo F, Thiry E, et al. Aetiology of canine infectious respiratory disease complex and prevalence of its pathogens in Europe. J Comp Pathol. 2020;176:86-108.
Sykes JE. Pediatric feline upper respiratory disease. Vet Clin North Am Small Anim Pract. 2014;44(2):331-342.
Costa MO, Lage B. Streptococcus equi subspecies zooepidemicus and sudden deaths in swine, Canada. Emerg Infect Dis. 2020;26(10):2522-2524.
Gruszynski K, Young A, Levine SJ, Garvin JP, Brown S, Turner L, et al. Streptococcus equi subsp. zooepidemicus infections associated with guinea pigs. Emerg Infect Dis. 2015;21(1):156-158.
Pisoni G, Zadoks RN, Vimercati C, Locatelli C, Zanoni MG, Moroni P. Epidemiological investigation of Streptococcus equi subspecies zooepidemicus involved in clinical mastitis in dairy goats. J Dairy Sci. 2009;92(3):943-951.
Corpa JM, Carvallo F, Anderson ML, Nyaoke AC, Moore JD, Uzal FA. Streptococcus equi subspecies zooepidemicus septicemia in alpacas: three cases and review of the literature. J Vet Diagn Invest. 2018;30(4):598-602.
Mätz-Rensing K, Winkelmann J, Becker T, Burckhardt I, van der Linden M, Köndgen S, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infection in a group of rhesus monkeys (Macaca mulatta). J Med Primatol. 2009;38(5):328-334.
EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, et al. Assessment of animal diseases caused by bacteria resistant to antimicrobials: Horses. EFSA J. 2021;19(12):1-43.
Samper JC, Tibary A. Disease transmission in horses. Theriogenology. 2006;66(3):551-559.
Bordes-Benítez A, Sánchez-Oñoro M, Suárez-Bordón P, García-Rojas AJ, Saéz-Nieto JA, González-García A, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infections on the island of Gran Canaria associated with the consumption of inadequately pasteurized cheese. Eur J Clin Microbiol Infect Dis. 2006;25(4):242-246.
Toraño G, Arias I, Castillo A, Brossard G. Primer caso de meningitis por Streptococcus equi subsp. zooepidemicus en Cuba. Rev Cub Salud Pública. 2015;41(1):165-168.
Høyer-Nielsen AK, Gaini S. Sepsis, endocarditis, and purulent arthritis due to a rare zoonotic infection with Streptococcus equi subspecies zooepidemicus. Case Rep Infect Dis. 2018;2018:1-8.
Zahlanie Y, Almatrafi M, Filkins L, Hsiang MS. Possible canine source of Streptococcus equi subspecies zooepidemicus causing meningitis in an infant. IDCases. 2019;17:1-3.
Nocera FP, D'Eletto E, Ambrosio M, Fiorito F. Occurrence and antimicrobial susceptibility profiles of Streptococcus equi subsp. zooepidemicus strains isolated from mares with fertility problems. Antibiotics. 2022;11(25):1-11.
Lord J, Carter C, Smith J, Locke S, Phillips E, Odoi A. Antimicrobial resistance among Streptococcus equi subspecies zooepidemicus and Rhodococcus equi isolated from equine specimens submitted to a diagnostic laboratory in Kentucky, USA. PeerJ. 2022;10:1-23.
Malaluang P, Wilén E, Frosth S. Vaginal bacteria in mares and the occurrence of antimicrobial resistance. Microorganisms. 2022;10(11):1-18.
Deniaud M, Tee E. Susceptibility pattern of bacterial isolates in equine ulcerative keratitis: Implications for empirical treatment at a university teaching hospital in Sydney. Aust Vet J. 2023;101(3):115-120.
Malaluang P, Wilén E, Lindahl J. Antimicrobial Resistance in Equine Reproduction. Animals. 2021;11:1-13.
Petersen MR, Skive B, Christoffersen M, Lu K, Nielsen JM, Troedsson MH, et al. Activation of persistent Streptococcus equi subspecies zooepidemicus in mares with subclinical endometritis. Vet Microbiol. 2015;179(1-2):119-125.
Boyle AG. Streptococcus equi subspecies equi. Vet Clin North Am Equine Pract. 2023;39(1):115-131.
Mani RJ, Thachil AJ, Ramachandran A. Discrimination of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Vet Diagn Invest. 2017;29(5):622-627.
Uchida-Fujii E, Niwa H, Kinoshita Y, Nukada T. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) for identification of bacterial isolates from horses. J Equine Vet Sci. 2020;95:1-7.
CLSI. VET08 Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 4th ed. Wayne, USA: Clinical and Laboratory Standards Institute; 2018. 200 p.
The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0. Available online: http://www.eucast.org: EUCAST; 2023. 110 p.
Awosile BB, Heider LC, Saab ME, McClure JT. Antimicrobial resistance in bacteria isolated from horses from the atlantic provinces, Canada (1994 to 2013). Can Vet J. 2018;59(9):951-957.
Kerdsin A, Chopjitt P. Zoonotic infection and clonal dissemination of Streptococcus equi subspecies zooepidemicus sequence type 194 isolated from humans in Thailand. Transbound Emerg Dis. 2022;69(4):554-565.
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Expert Rules. Streptococcus. Available online: http://www.eucast.org: EUCAST; 2019. 3 p.
Léon A, Castagnet S, Maillard K, Paillot R, Giard JC. Evolution of in vitro antimicrobial susceptibility of equine clinical isolates in France between 2016 and 2019. Animals. 2020;10(812):1-11.
Jospe-Kaufman M, Siomin L, Fridman M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg Med Chem Lett. 2020;30(13):1-6.
Haenni M, Lupo A, Madec JY. Antimicrobial resistance in Streptococcus spp. Microbiol Spectr. 2018;6(2):1-25.
World Health Organization & WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically important antimicrobials for human medicine: Ranking of antimicrobial agents for risk management of antimicrobial resistance due to non-human use. 6th ed. Geneva, Switzerland: World Health Organization; 2019. 52 p.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281.
Sweeney MT, Lubbers BV, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother. 2018;73(6):1460-1463.