Mollicutes associated with mastitis in dairy cattle herds in Zamora-Chinchipe province, Ecuador
Main Article Content
Abstract
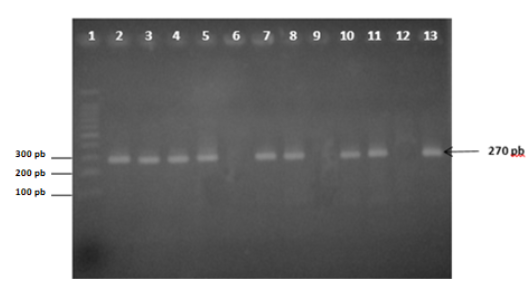
World population growth demands availability of sufficient nutritious and healthy food. Bovine mastitis is a disease that still causes great economic losses, besides other risks to the health of herds and people in general, in spite of the technological advances and the deepening of the knowledge of crucial factors in its behavior, which depend on the hosts and pathogens involved and the environment where they interact.To further research Mollicutes association with mastitis disease in Zamora-Chinchipe province, 386 cows from 99 herds were tested. From them, 126 were positive to Mollicutes (32.6 %) by Polymerase Chain Reaction (PCR), while 340 (88.1 %) were found to have subclinical mastitis by California Mastitis Test. Although Mycoplasma bovis was not detected, other pathogenic mycoplasmas could also be present affecting the recovery of the infected herds due to the recognized resistance of those microorganisms to the traditional antibiotic therapy, among other risks. The lack of association among the results of the diagnostic methods used showed that other pathogens were involved in mastitis etiology and revealed the need for further studies in order to adequately guide control strategies.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
National Center for Animal and Plant Health (CENSA)References
Zadoks RN, Middleton JR, McDougal S, Katholm J, Schukken YH. Molecular Epidemiology of Mastitis Pathogens of Dairy Cattle and Comparative Relevance to Humans. Mammary Gland Biol Neoplasia. 2011; 16:357-372. DOI 10.1007/s10911-011-9236-y
Boboš S, Radinovic´ M, Vidic´ B, Pajic´ M, Vidic´ V, Galfi A. Mastitis therapy: Direct and indirect costs. Biotechnol. Anim. Husb. 2013; 29, 269-275
Hertl J, Shukken Y, Bar D, Bennett G, González R, Rauch B, et al. The effect of recurrent episodes of clinical mastitis caused by Gram-positive and Gram-negative bacterias and other organism on mortality and culling in Holstein dairy cows. J. Dairy Sci. 2011; 94:4863-4877.
Fernandes I, Guimaraes NR, Noyes LS, Caixeta VS. Effect of subclinical mastitis detected in the first month of lactation on somatic cell count linear scores, milk yield, fertility, and culling of dairy cows in certified organic herds, Journal of Dairy Science. 2021; 104 (2): 2140-2150. https://doi.org/10.3168/jds.2020-19153.
da Silva Bomfim BV, Costa Balieiro A, Messias de Souza Ferreira da Costa AC, Teles Romeiro E, de Souza Franco E, Menezes Wanderley ML et al. Factores de riesgo asociados a la mastitis bovina. Rev. Ibero-Am. de Hum., Ciências e Educ. São Paulo. 2023; 9(3). REASE. http://doi.org/10.51891/rease.v9i3.8958
Awandkar SP, Kulkarni MB, Khode NV. Bacteria from bovine clinical mastitis showed multiple drug resistance. Veterinary Research Communications. 2022; 46(1), 147-158.
Paramasivam R, Raj Gopal D, Dhandapani R, Subbarayalu R, Prabu Elangovan M y col. Is AMR in Dairy Products a Threat to Human Health? An Updated Review on the Origin, Prevention, Treatment, and Economic Impacts of Subclinical Mastitis. Infection and Drug Resistance. 2023; 16.
Dhakal K, Tiezzi F, Clay JS, Maltecca C. Causal relationships between clinical mastitis events, milk yields and lactation persistency in US Holsteins. Livestock Science. 2016; 189, 8-16.
Citti C, Blanchard A. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends in Microbiology. 2013; 21(4):196-203. https://doi.org/10.1016/j.tim.2013.01.003
Ruegg PL. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017; 100:10381-10397. https://doi.org/10.3168/jds.2017-13023
Haapala V, Vähänikkilä N, Kulkas L, Tuunainen E, Pohjanvirta T, Autio T, et al. Mycoplasma bovis infection in dairy herds-Risk factors and effect of control measures. J. Dairy Sci. 2021; 104:2254-2265 https://doi.org/10.3168/jds.2020-18814
Justice-Allen A, Trujillo J, Goodell G, Wilson D. Detection of multiple Mycoplasma species in bulk tank milk samples using real-time PCR and conventional culture and comparison of test sensitivities. J Dairy Sci. 2011; 94:34113419
Adorno, BMV, Salina A, Joaquim SF, Guimarães FF, Lopes BC, Menozzi BD, Langoni H. Presença de Mollicutes e Mycoplasma bovis em Swabs Nasais de bezerros e na secreção mastítica de vacas Vet. e Zootec. 2021; 28: 001-009. ISSN Eletrônico 2178-3764
Deeney AS, Collins R, Ridley AM. Identification of Mycoplasma species and related organisms from ruminants in England and Wales during 2005-2019. BMC Veterinary Research. 2021; 17(1):1-12.
Ramírez-Sanmartín N, Luis Rodrigo-Saa, Percedo-Abreu MI, Pérez Castillo A, Lobo-Rivero E. Detección de Mollicutes en leche de tanques procedentes de la provincia Zamora-Chinchipe, Ecuador. Rev. Salud Anim. 2017; 39(3)
Hervada Vidal X, Naveira Barbeito G, Santiago Pérez MI, Mujica Lengua ÓJ, Vázquez Fernández E, Manrique Hernández R et al. Epidat: programa para análisis epidemiológico de datos. Consellería de Sanidade, Xunta de Galicia, España, Organización Panamericana de la Salud (OPS-OMS), 2016. Universidad CES, Colombia.
Saa LR, Perea A, García-Bocanegra I, Arenas AJ, Jara DV, Ramos R, Carbonero A. Seroprevalence and risk factors associated with bovine viral diarrhea virus (BVDV) infection in non-vaccinated dairy and dual purpose cattle herds in Ecuador. Trop Anim Health Prod. 2012; 44:645-649. DOI 10.1007/s11250-011-9948-4
National Mastitis Council (NMC). Golbal Milk Quality. Procedures for Collecting Milk Samples. 2014; http://www.nmconline.org/sampling.htm
Rossetti B, Frey J, Pilo P. Direct detection of Mycoplasma bovis in milk and tissue samples by real-time PCR. Mol Cell Probes. 2010; 24:321-323.
Van Kuppeveld FJM, Johansson KE, Galama JMD, Kissing J, Bölske G, van der Logt JTM et al. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Applied Environment Microbiol.1998; 60:149-152.
Hotzel H, Heller M, Sachse K. Pfützner H. Rapid detection of Mycoplasma bovis in milk samples and nasal swabs using the polymerase chain reaction. Mol Cell Probes. 1996; 13:175e8
Blowey R, Edmondson P. Somatic cell count. Mastitis Control in dairy herds, (Ed. 2). 2010; 152-170.
Castillo Duvergel Y, Miranda I. COMPAPROP: Sistema para comparación de proporciones múltiples. Rev. Protección Veg. 2014; 29(3): 231-234
Amer S, Gálvez FLA, Fukuda Y, Tada C, Ludeña IL, Valle WFM, Nakai Y. Prevalence and etiology of mastitis in dairy cattle in El Oro Province, Ecuador. J. Vet. Med. Sci. 2018; 80(6):861-868. DOI:10.1292/jvms.17-0504
Cuenca M, García D, Reinoso L, González J, Torracchi J. Detección de Mastitis Subclínica Bovina y factores asociados, en fincas lecheras de la Provincia del Cañar--Biblián, Ecuador. Revista Científica de la Facultad de Ciencias Veterinarias. 2021; 31(3), 93-98.https://doi.org/10.52973/rcfcv-luz313.art3
Wilson DJ, Skirpstunas RT, Trujillo JD, Cavende KB, Bagley CV, Harding RL. Unusual history and initial clinical signs of Mycoplasma bovis mastitis and arthritis in first lactation cows in a closed commercial dairy herd. J Am Vet Med Assoc. 2007; 230:1519-1523.
Kruze J, Mella A. First bovine clinical mastitis outbreak in a large dairy herd in Chile caused by the environmental algae P. zopfii. In Proc XXVI World Buiatrics Congress. 2011
Gioia G, Addis MF, Santisteban C, Gross B, Nydam DB, Sipka AS et al. Mycoplasma species isolated from bovine milk collected from US dairy herds between 2016 and 2019. J. Dairy Sci. 2020; 104:4813-4821. https://doi.org/10.3168/jds.2020-19171
Parker AM, Sheehy PA, Hazelton MS, Bosward KL, House JK. A review of mycoplasma diagnostics in cattle. J Vet Intern Med. 2018; 32:1241-1252. https://doi.org/10.1111/jvim.15135
Manzi MP, Joaquim SF, Guimarães FF, Bruder-Nascimento ACMO, Pantoja JC, Langoni H. Prevalência de Mycoplasma bovis em rebanhos de vacas leiteiras. Pesq. Vet. Bras. 2018; 38(4):665-669. DOI: 10.1590/1678-5150-PVB-5192
Nunes de Morais AC, Regis Pires D, Costa da Cunha N, dos Santos Machado L, Abdalla Helayel M, Monteiro de Mendonça JF et al. Risk factors associated with intramammary colonization with Mollicutes in dairy cattle from Southeast Brazil. Ciência Rural, Santa Maria. 2021; 51:8 http://doi.org/10.1590/0103-8478cr20200694
Salina A, Timenetsky J, Barbosa MS, Azevedo CM, Langoni H. Microbiological and molecular detection of Mycoplasma bovis in milk samples from bovine clinical mastitis. Pesq. Vet. Bras. 2020; 40(2):82-87.
Andrade-Becerra, R. J., Caro-Carvajal, Z., Pulido-Medellín, M., López-Cepeda, M. Prevalencia de Mycoplasma spp., en fincas lecheras del Altiplano Boyacense (Colombia). Revista UDCA Actualidad & Divulgación Científica. 2014; 17(2), 461-466.
Ulloa, F., Soto, J.P., Kruzea, J., Mella, A. Mycoplasma isolation in milk samples from dairy herds in Chile. Austral J Vet Sci. 2021; 53:109-113
Maunsell F, Donovan A. Mycoplasma bovis Infections in young calves. Vet Clin Food Anim. 2009; 25:139-177.
Hazelton MS, Morton JM, Parker AM, Bosward KL, Sheehy A, Dwyer CJ, et al. Mycoplasma bovis and other Mollicutes in replacement dairy heifers from Mycoplasma bovis-infected and uninfected herds: A 2-year longitudinal study. J. Dairy Sci. 2020; 103:11844-11856. https://doi.org/10.3168/jds.2020-18921
Barkema HW, Schukken YH, Zadoks RN. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006; 89:1877-1895.
De Schutter L. Mycoplasma bovis alsveroorzaker van acute uierontsteking. Epidemiologis chonderzoek op 2 positieve melk veebedrijv en Katholieke Hoge school Kempen, Campus Geel, Belgium. 2010
Maunsell FP, Woolums AR, Francoz D, Rosenbusch RF, Step DL, Wilson DJ, Janzen ED. Mycoplasma bovis Infections in Cattle. J Vet Intern Med. 2011; 25:772-783
Fujimoto Y, Ito H, Higuchi H, Ohno H, Makita K. A case-control study of herd- and cow-level risk factors associated with an outbreak of Mycoplasma mastitis in Nemuro, Japan. Preventive Veterinary Med. 2020; 177, 104946. https://doi.org/10.1016/j.prevetmed.2020.104946
Dudek, K.; Szacawa, E.; Nicholas, R.A.J. Recent Developments in Vaccines for Bovine Mycoplasmoses Caused by Mycoplasma bovis and Mycoplasma mycoides subsp. mycoides. Vaccines. 2021, 9, 549. https://doi.org/10.3390/vaccines9060549