Efecto de la interacción entre dos especies de miridos depredadores (Hemiptera: Miridae) en la presa Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)
Contenido principal del artículo
Resumen
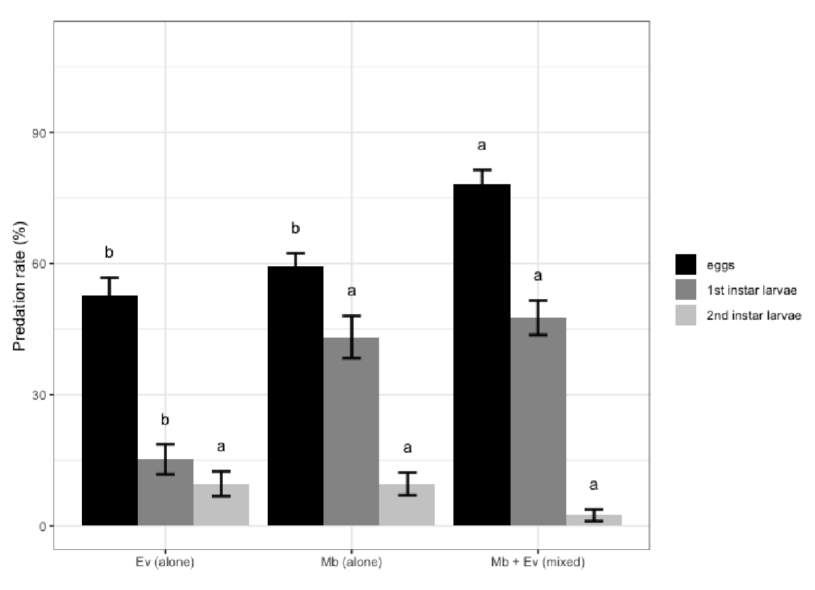
Los estudios sobre sistemas con múltiples especies depredadoras han demostrado que las interacciones entre especies pueden no ser predecibles y dependen, en gran medida, de los rasgos de comportamiento individuales, la densidad de las especies y la complejidad del hábitat. Se examinaron las interacciones de dos míridos depredadores: Macrolophus basicornis (Stal) y Engytatus varians (Distant) (Hemiptera: Miridae) con la plaga Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), estimando los efectos positivos o negativos de su combinación mediante un modelo de riesgo multiplicativo (MRM). Se estimó la eficacia de las especies de míridos, individualmente y mixtas, contra los huevos y las larvas de la plaga, mediante las tasas de depredación durante 24 horas en condiciones de laboratorio. Ambas especies de míridos prefirieron los huevos y las larvas de primer estadio de T. absoluta. Su combinación fue positiva al alimentarse de estos estadios de la plaga, pero negativa en el segundo estadio larvario. Los resultados, basados en las tasas de depredación, mostraron que M. basicornis tiene una mayor capacidad de depredación en comparación con E. varians sobre las larvas de primer estadio de T. absoluta, pero ambos míridos son buenos candidatos como agentes de control biológico de T. absoluta en el cultivo del tomate.
Detalles del artículo

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra, siempre que se indique su autor y la primera publicación en esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
Citas
Guedes RNC, Picanço MC. The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. Bulletin OEPP/EPPO. 2012;42:211-216. https://doi.org/10.1111/epp.2557
Urbaneja A, Vercher R, Navarro V, García MF, Porcuna JL. La polilla del tomate, Tuta absoluta. Phytoma España. 2007;194:16-23.
Toševski I, Jović J, Mitrović M, Cvrković T, Krstić O, Krnjajić S. Tuta absoluta (Meyrick, 1917) (Lepidoptera, Gelechiidae): a New Pest of Tomato in Serbia. Pesticides & Phytomedicine. 2011;3:197-204. https://doi.org/10.2298/PIF1103197T
Biondi A, Guedes NRC, Wan F, Desneux N. Ecology, worldwide spread, and management of the invasive south american tomato pinworm, Tuta absoluta: Past, present, and future. Annual Review of Entomology. 2018;63:239-58. https://doi.org/10.1146/annurevento-031616-034933
CABI Head Office, Wallingford, UK. Distribution Maps of Plant Pests, Tuta absoluta. [Distribution map]. Map 723(1st revision). 2016. Consulted: 29/3/2018 available online: http://www.cabi.org/isc/datasheet/49260
Verheggen F, Bertin R. First record of Tuta absoluta in Haiti. Entomologia Generalis. 2019; 38:349-353. https://doi.org/10.1127/entomologia/2019/0778
Bueno VHP, van Lenteren JC, Lins Jr, Calixto AM, Montes F, Silva D, et al. New records of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) predation by Brazilian Hemipteran predatory bugs. Journal Applied Entomology. 2013;137:29-34. https://doi.org/10.1111/jen.12017
Han P, Bayram Y, Shaltiel‐Harpaz L, Sohrabi F, Saji A, Esenali UT, et al. Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. Journal of Pest Science. 2018. https://doi.org/10.1007/s10340-018-1062-1
Haddi K, Berger M, Bielza P, Rapisarda C, Williamson MS, Moores G, et al. Mutation in the ace-1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. Journal Applied Entomology. 2017. https://doi.org/10.1111/jen.12386
Biondi A, Guedes NRC, Wan F, Desneux N. Ecology, worldwide spread, and management of the invasive south american tomato pin- worm, Tuta absoluta: Past, present, and future. Annu Rev Entomol. 2018;63:239-258 https://doi.org/10.1146/annurevento-031616-034933
Siqueira HA, Guedes RN, Picanco MC. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agricultural Entomology. 2000;2:147-153.
Biondi A, Zappalà L, Stark JD, Desneux N. Do Biopesticides Affect the Demographic Traits of a Parasitoid Wasp and Its Biocontrol Services through Sublethal Effects? PLoS ONE. 2013;9: e76548. https://doi.org/10.1371/journal.pone.0076548
Zappalà L, Siscaro G, Biondi A, Mollá O, González‐Cabrera J, Urbaneja A. Efficacy of sulphur on Tuta absoluta and its side effects on the predator Nesidiocoris tenuis. Journal of Applied Entomology. 2012;6:401-409. https://doi.org/10.1111/j.1439-0418.2011.01662.x
Calvo FJ, Lorente MJ, Stansly PA, Belda JE. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisia tabaci in greenhouse tomato. Entomologia Experimetalis et Applicata. 2012;143:111-119 https://doi.org/10.1111/j.1570-7458.2012.01238.x
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl. 2018a;63:39-59. https://doi.org/10.1007/s 10526-017-9801-4.
Urbaneja A, Monton H, Mollá O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. Journal Applied Entomology. 2009;133:292-296. https://doi.org/10.1111/j.1439-0418.2008.01319.x
Moreno-Ripoll R, Agustí N, Berruezo R, Gabarra R. Conspecific and heterospecific interactions between two omnivorous predators on tomato. Biological Control. 2012;62:189-196. https://doi.org/10.1016/j.biocontrol.2012.04.005
Lampropoulos P, Perdikis D, Fantinou A. Are multiple predator effects directed by prey availability? Basic and Applied Ecology. 2013;14:605-613. https://doi.org/10.1016/j.baae.2013.08.004
Perdikis D, Lucas E, Garantonakis N, Giatropoulos A, Kitsis P, Maselou D, et al. Intraguild predation and sublethal interactions between two zoophytophagous mirids, Macrolophus pygmaeus and Nesidiocoris tenuis. Biological Control. 2014;70:35-41. https://doi.org/10.1016/j.biocontrol.2013.12.003
Ferreira PS, da Silva E, Coelho LB. Miridae (Heteroptera) fitófagos e predadores de Minas Gerais, Brasil, com ênfase em espécies com potencial econômico. Iheringia, Série Zoologia. 2001;91:159-169.
Silva DB, Bueno VHP, Montes FC, van Lenteren JC. Population growth of three mirid predatory bugs feeding on eggs and larvae of Tuta absoluta on tomato. BioControl. 2016. https://doi.org/10.1007/s10526-016-9736-1
van Lenteren JC, Hemerik L, Lins Jr and Bueno VHP. Functional Responses of Three Neotropical Mirid Predators to Eggs of Tuta absoluta on Tomato. Insects. 2016;7:34. https://doi.org/10.3390/insects7030034.
van Lenteren JC, Bueno VHP, Calvo FJ, Calixto AM, Montes FC. Comparative effectiveness and injury to tomato plants of three Neotropical mirid predators of Tuta absoluta (Lepidoptera: Gelechiidae). Journal of Economic Entomology. 2018b. https://doi.org/10.1093/jee/toy057
van Lenteren JC, Bueno VHP, Burgio G, Lanzoni A, Montes FC, Silva D, et al. Pest kill rate as aggregate evaluation criterion to rank biological control agents:a case study with Neotropical predators of Tuta absoluta on tomato. Bulletin of Entomological Research. 2019. https://doi.org/10.1017/S0007485319000130
Martínez LD, Martínez Rivero MA, Bueno VHP, Collatz J. Predation behaviour and prey preference of two neotropical mirids against two key lepidopteran pests in tomato. International Journal of Tropical Insect Science 2021. https://doi.org/10.1007/s42690-021-00605-5
Martínez MA, Duarte L, Baños HL, Rivas A, Sánchez A. Predatory mirids (Hemiptera: Heteroptera: Miridae) in tomato and tobacco in Cuba. Rev. Protección Veg. 2014;3:204-207
Furlong MJ. Knowing your enemies: Integrating molecular and ecological methods to assess the impact of arthropod predators on crop pests. Insect Science. 2014;22:6-19. https://doi.org/10.1111/1744-7917.12157
Sih A, Englund G, Wooster D. Emergent impacts of multiple predators on prey. Trends in Ecology & Evolution. 1998;13:350-355.
Soluk DA, Collins NC. Synergistic interactions between fish and stoneflies: Facilitation and interference among stream predators. Oikos. 1988;52:94-100.
Giustolini TA, Vendramin JD, Parra JRP. Número de instares larvais de Tuta absoluta (Meyrick) em genótipos de tomateiro. Scientiae Agricola. 2002;59:393-6.
Soluk DA. Multiple prey effects: Predicting mixed functional response of stream fish and invertebrate predators. Ecology. 1993;74:19-225.
Griffen B. Detecting emergent effects of multiple predator species. Oecologia. 2006;148:702-709. https://doi.org/10.1007/s00442-006-0414-3
Schmitz OJ. Predator diversity and trophic interactions. Ecology. 2007;88:2415-2426
R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. URL https://www.R-project.org/
Urbaneja A, González-Cabrera J, Arnó J, Gabarra R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Management Science. 2012;68:1215-1222. https://doi.org/10.1002/ps.3344.
McCoy W, Stier C, Osenberg W. Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecology Letters. 2012;15:1449- 1456. https://doi.org/10.1111/ele.12005
Tabic A, Yonah R, Coll M. Association between omnivorous Orius bugs and their thrips prey at different spatial scales of Verbesina encelioides flowers. Israel Journal of Plant Sciences. 2010;58:131-141. https://doi.org/10.1560/IJPS.58.2.131
Belliure B, Amorós-Jiménez R, Fereres A, Marcos-García MA. Antipredator behaviour of Myzus persicae affects transmission efficiency of Broad bean wiltvirus 1. Virus Research. 2011;159:206-214.
Duarte L, Pacheco R, Quiñones M, Martínez MA, Bueno VHP. Nesidiocoris tenuis Reuter (Hemiptera: Miridae) and Cycloneda sanguinea limbifer (Casey) (Coleoptera: Coccinelidae): Behaviour and predatory activity on Myzus persicae Zulzer (Hemiptera: Aphididae). Revi. Protección Veg. 2014;29:99-105
Olson RS, Hintze A, Dyer FC, Knoester DB, Adami C. Predator confusion is sufficient to evolve swarming behaviour. Journal of the Royal Society Interface. 2013;10:0305. https://doi.org/10.1098/rsif.2013.0305
Tylianakis JM, Rand TA, Kahmen A, Klein AM, Buchmann N, Perner J, et al. Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLoS Biology. 2008;6(5):e122. https://doi.org/10.1371/journal.pbio.0060122.
Tylianakis JM, Romo CM. Natural enemy diversity and biological control: Making sense of the context-dependency. Basic and Applied Ecology. 2010;11:657-668. https://doi.org/10.1016/j.baae.2010.08.005.
Cusumano A, Peri E, Vinson SB, Colazza S. Intraguild interactions between two egg parasitoids exploring host patches. BioControl. 2011;56:173-184. https://doi.org/10.1007/s10526-010-9320-z.
Rondoni G, Onofri A, Ricci C. Differential susceptibility in a specialized aphidophagous ladybird, Platynaspisluteo rubra (Coleoptera: Coccinellidae), facing intraguild predation by exotic and native generalist predators. Biocontrol Science and Technology. 2012;2211:1334-1350. https://doi.org/10.1080/09583157.2012.726607
Gagnon AE, Heimpel GE, Brodeur J. The Ubiquity of Intraguild Predation among Predatory 474 Arthropods. PLoS ONE. 2011;11:e28061. 475 https://doi.org/10.1371/journal.pone.0028061