Morphological-cultural characterization of fungi associated with the fruits of chonta (Bactris gasipaes Kunth), in five locations of Orellana, Ecuador
Main Article Content
Abstract
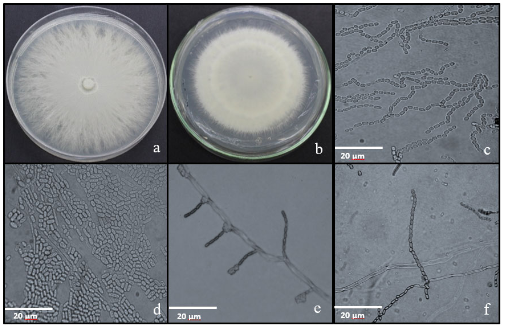
The aim of this study was to isolate and identify the fungi associated with chonta fruits in five localities belonging to the province of Orellana. During the month of February2020, 22 samples of chonta fruits of different phenotypes with symptoms were collected in the localities of San Francisco de Asis, El Coca, Sani Isla, Patasyaku, and San José. The isolation of the fungi associated with the diseased fruits was carried out by the direct method on Potato-Dextrose-Agar and Sabouraud-Dextrose-Agar culture media. In addition, the colonies were grouped into morphotypes on the basis of macroscopic characteristics. The morphotypes were identified by morphometric characterization taking into account the criteria described in the scientific literature. The fungi associated with chonta fruits belonged to the genera Geotrichum, Mucor, Cladosporium, Fusarium, Trichoderma, and Ceratocystis
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra, siempre que se indique su autor y la primera publicación en esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
References
Ríos D, Castillo E, Fuchs E. Estado actual del banco de germoplasma de pejibaye (Bactris gasipaes), Guápiles, Costa Rica. Agronomía Mesoamericana. 2016; 27(2):311-317.
PROECUADOR. Consumo de palmito en Estados Unidos, 2018. Disponible en: https://www.proecuador.gob.ec/consumo-de-palmito-en-estados-unidos/ . [Consulta: 12 julio 2021].
Montúfar R, Rosas J. Chontaduro/Chontilla [en línea]. En: Valencia R, Montúfar R, Navarrete H, Balslev H (Eds.). Palmas Ecuatorianas: biología y uso sostenible. Publicaciones del Herbario de la Pontificia Universidad Católica del Ecuador. 2013; p. 63-89. Disponible en: https://issuu.com/juanlorenzo/docs/palmas_ecuador [Consulta: 23 junio 2020].
Arroyo C, Arauz L, Mora, J. Incidencia de enfermedades en pejibaye (Bactris gasipaes Kunth) para palmito. Agronomía Mesoamericana. 2004; 15(1):61-68.
Vida JB, Tessmann DJ, Mafacioli R, et al. Colletrotrichum gloeosporioides causando antracnose em frutos de pupunheira nos estados de Minas Gerais e Paraná. Summa Phytopathologica. 2006; 32(4): 379-380.
Cerón C, Palacios W, Guevara J, et al. Ministerio del Ambiente del Ecuador. Sistema de Clasificación de los Ecosistemas del Ecuador Continental [en línea]. Quito-Ecuador: Ministerio del Ambiente del Ecuador. [Consulta: 20 julio 2020], 2013. Disponible en: http://app.sni.gob.ec/snilink/sni/PDOT/NIVEL%20NACIONAL/MAE/ECOSISTEMAS/DOCUMENTOS/Sistema.pdf
Alfenas AC, Mafia RG. Métodos de Fitopatología. 2a Ed. Universidad Federal de Viçosa. Viçosa, Brasil: Editora UFV. 2016; 59-63.
Balmas V, Santori A, Corazza L. Le specie di Fusarium piú comuni in Italia. Suggerimenti per il loro riconoscimento. Istituto Sperimentale per la Patologia Vegetale. Petria Giornale di Patología delle Piante. 2000; 10(1):1-60.
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi.4a Ed. Saint Paul, Minnesota-United States of America: The American Phytopathological Society. 1998;1-237.
Benson H. Microbiological Applications Laboratory Manual in General Microbiology. 8a Ed. New York, United States of America: Editora McGraw-Hill Science/Engineering/Math. 2001: 99-102 y 157-160.
Leslie JF, Summerell BA. The Fusarium Laboratory Manual. Garsington Road, Oxford- United Kingdom. Photographs by Suzanne Bullock: Blackwell Publishing. 2006: 80-272.
Munsell C. Munsell Color Charts for Plant Tissues. New Windsor, New York-United States of America: Munsell Color, 1997.
Larone D, Walsh T, Hayden R. Larone's Medically Important Fungi: A Guide to Identification. 6th ed. Washington, United States of America. DC: ASM Press, 2018; p. 73-331.
Ferraz L, Da Cunha T, Da Silva A, et al. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit”. Microbiological Research. 2016: 72-79. Doi: https://doi.org/10.1016/j.micres.2016.04.012
Wanjiku EK, Waceke JW, Wanjala BW, et al. Identification and Pathogenicity of Fungal Pathogens Associated with Stem End Rots of Avocado Fruits in Kenya. Rev International Journal of Microbiology. 2020: 1-8. Doi: https://doi.org/10.1155/2020/4063697.
Jayawardena RS, Hyde KD, Chen YJ, et al. One stop shop IV: taxonomic update with molecular phylogeny for important phytopathogenic genera: Fungal Diversity [en línea], (Netherlands). 2020: 76-218. Doi: https://doi.org/10.1007/s13225-020-00460-8
Cruz I, García R, Carillo J, et al. Identification of mucorales an fungi causing soft rot in papaya (Carica papaya L.) fruit in Mexico. Revista Mexicana de Fitopatología. 2017; 35 (3): 397-Doi: https://doi.org/10.18781/r.mex.fit.1611-3
Hyde KD, Nilsson RH, Alias SA, et al. One stop shop: backbones trees for important phytopathogenic genera: Fungal Diversity [enlínea], (Netherlands). 2014; 67: 21-125. Doi: https://doi.org/10.1007/s13225-014-0298-1
Bensch K, Braun U, Groenewald JZ, et al. The genus Cladosporium. Studies in Mycology. 2012; p. 1-401. Doi: https://doi.org/10.3114/sim0003
Pinho D, Dutra D, Pereira O. Notes on Ceratocystis paradoxa causing internal post-harvest rot disease on immature coconut in Brazil. Tropical Plant Pathology. 2013; 38 (2): 152-157. Doi: https://www.scielo.br/pdf/tpp/v38n2/a10v38n2.pdf
De Melo MP, Da Silva MK, Júnior JE, et al. Thielaviopsis musarum causes postharvest crown and fruit rot of banana in Northeastern Brazil. Tropical Plant Pathology. 2016; 41: 258-263. Doi: https://doi.org/10.1007/s40858-016-0094-4
Crane J. Pejibaye (Peach Palm) Growing in the Florida Home Landscape [en línea]. United States of America: University of Florida. HS1072, 2016; p. 1-5. Doi: https://edis.ifas.ufl.edu/pdffiles/HS/HS31200.pdf
Santos P, Mussi V, Machado M. et al. Diagrammatic scale of severity for postharvest black rot (Ceratocystis paradoxa) in coconut palm fruits. Summa Phytopathologica. 2017; 43 (4): 269-275. Doi: https://doi.org/10.1590/0100-5405/170792
Rifai A. A revision of the genus Trichoderma. Mycol. Pap. 1969; 116:1-56.
Gams W, Bisset J. Morphology and identification of Trichoderma. En: C. Kubicek; G. Harman (eds.). 1998; p. 3- 34, Trichoderma and Gliocladium Volume 1: Basic biology, taxonomy and genetics. Taylor & Francis, London, UK.
Samuels GJ, Hebbar PK. Trichoderma: Identification and Agricultural Applications. United States of America: The American Phytopathological Society (APS). 2015; p. 49-71.
Dávila F. Diversidad de hongos asociados a lesiones foliares en naranjilla (Solanum quitoense Lam.) en varias localidades de la Amazonía Ecuatoriana. [Tesis de grado en Microbiología]. Pontificia Universidad Católica del Ecuador, Quito, Ecuador, 2015; p. 25-46. Doi: http://repositorio.puce.edu.ec/handle/22000/12524
Sapareng S, Ala A, Kuswinanti T, et al. Isolation and Characteristics Molding Fungus of Stalks and Empty Fruit Bunches of Oil Palms. International Journal of Scientific & Technology Research. 2018; 7 (11): 135-140.
Álvarez A, Nishijima W. Postharvest diseases of papaya. The American Phytopathological Society. 1987; 71 (8): 681-686.